High-throughput, physiologically relevant ion channel characterization using RealDRGx™ sensory neurons
Translational Relevance of iPSC-Derived Sensory Neurons
To address the critical need for scalable and human-relevant models in pain research, we validated an automated patch clamp platform using RealDRGx™ iPSC-derived sensory neurons, which offer native-like electrophysiological behaviour and compatibility with high-throughput workflows. This platform is ideal for studying sensory neuron excitability and screening novel pharmacological agents targeting inflammatory and neuropathic pain. Dorsal root ganglion (DRG) neurons are key mediators of nociceptive signalling and are central to the development of analgesics. However, traditional primary neuron models are limited by variability and low throughput. Our iPSC-derived DRG system enables a reproducible, scalable alternative for preclinical screening.
Six-Week Electrophysiological Profiling of RealDRGx™ Neurons
RealDRGx™ neurons consistently met quality control benchmarks, demonstrating high assay success rates and stable biophysical properties from week 3 onward—supporting confident integration into screening cascades. Over six weeks of maturation, we monitored:
- Seal resistance: Consistently >200 MΩ
- Series resistance: Stable and <20 MΩ
- Cell catch rate: >90% by week 3
- Capacitance: Steady values, indicating membrane stability
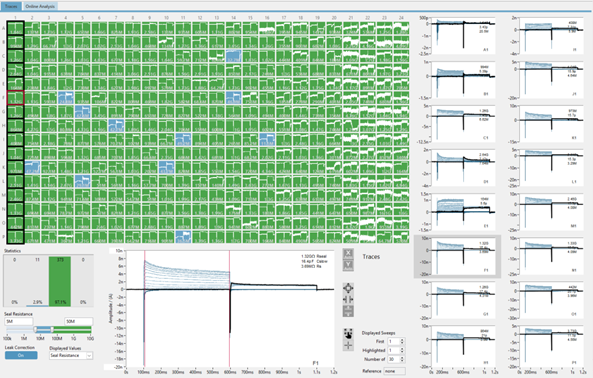
High-Throughput Electrophysiology
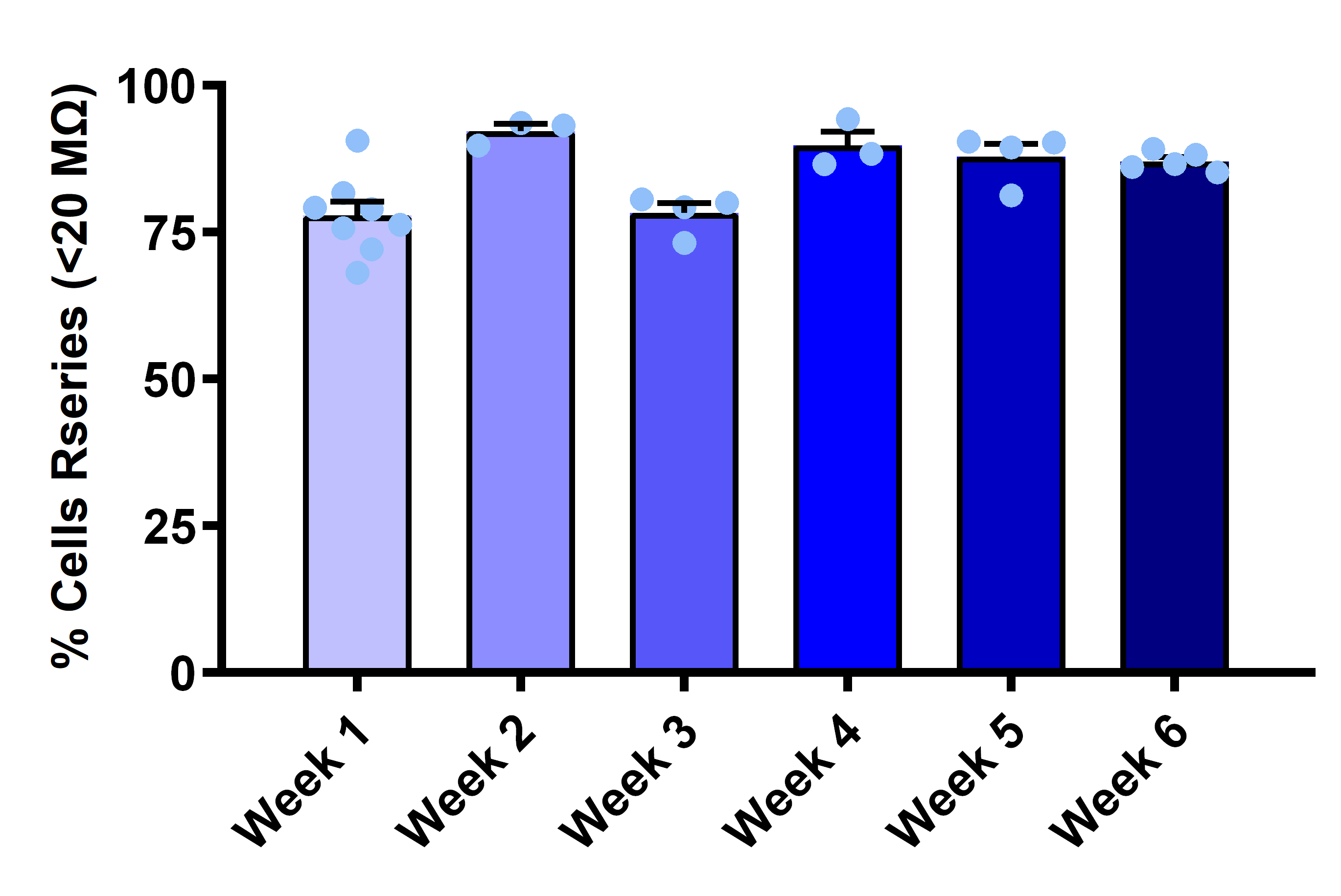
Consistent Low Series Resistance
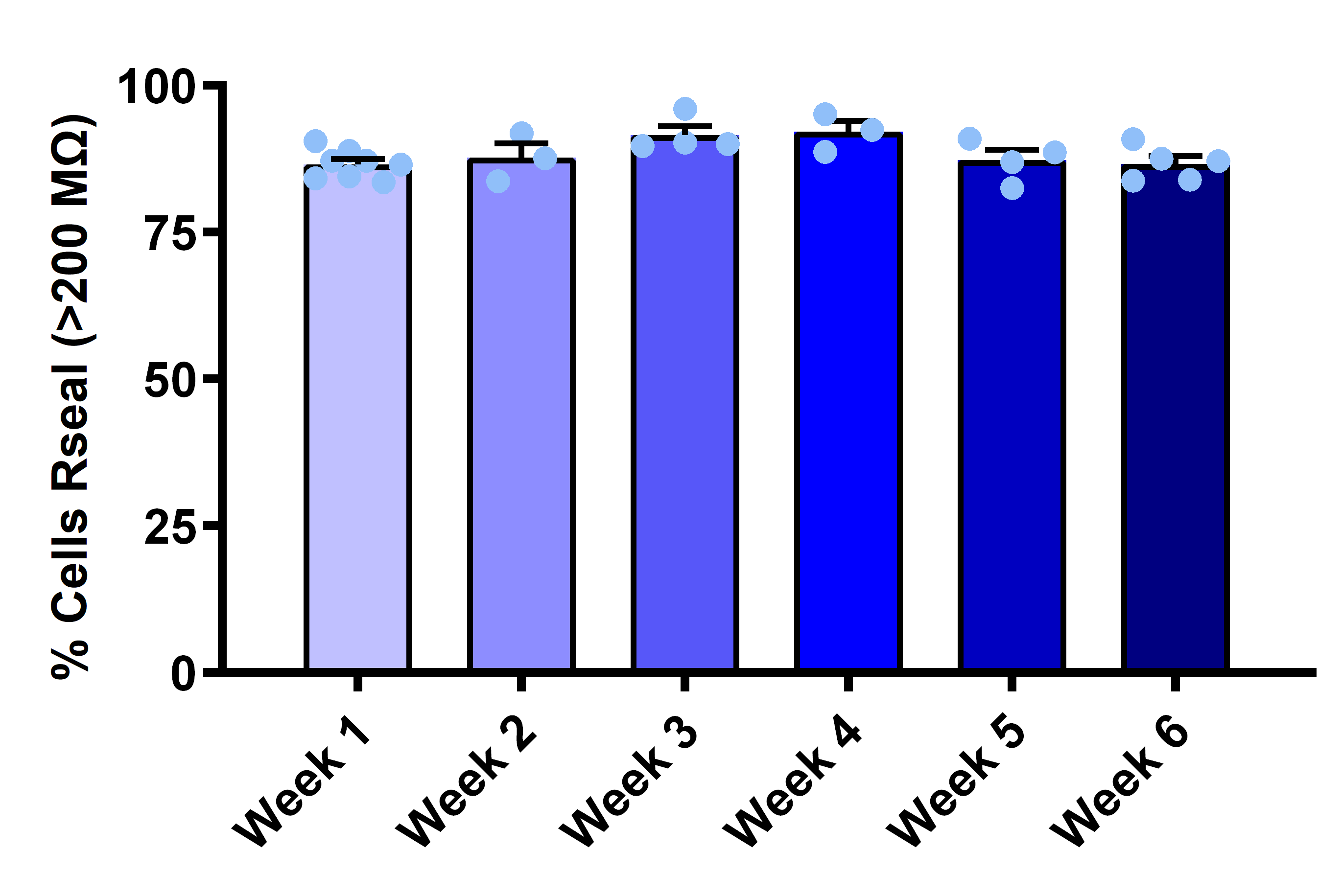
Reliable Seal Formation
Pharmacological Sensitivity Across Ion Channel Classes
Studies confirmed that RealDRGx™ sensory neurons express key voltage- and ligand-gated ion channels and respond reliably to standard tool compounds, supporting their use in pharmacological screening workflows. These results confirm functional expression and compound-responsiveness across targets relevant to pain signalling.
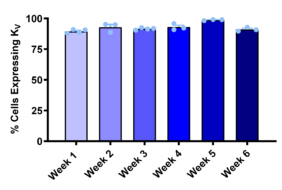
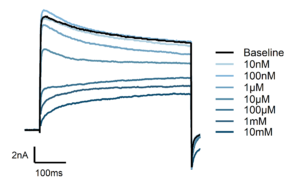
Figure 1: Functional Potassium Channel Expression and Pharmacological Modulation in iPSC-Derived DRG Neurons
RealDRGx™ sensory neurons demonstrate robust and sustained expression of functional voltage-gated potassium (Kv) channels, with over 90% of cell exhibiting Kv currents consistently from week 1 through week 6 (left). Cumulative current traces (right) show dose-dependent inhibition of Kv-mediated outward currents following application of 4-aminopyridine (4-AP), confirming pharmacological responsiveness and supporting their utility in ion channel screening workflows.


Figure 2: Sustained Sodium Channel Expression and TTX-Mediated Inhibition in iPSC-Drived DRG Neurons
iPSC-derived DRG neurons exhibited stable expression of voltage-gated sodium (Nav) channels, with over 90% of cells showing functional sodium currents across 6 weeks (left). Representative current traces (right) illustrate dose-dependent inhibition of sodium currents by tetrodotoxin (TTX), confirming pharmacological responsiveness and functional Nav channel activity in the model system.
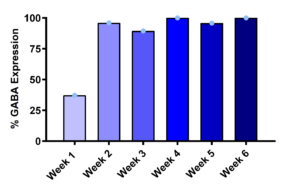
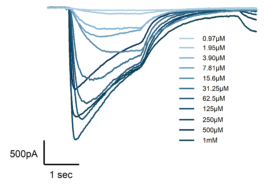
Figure 3: Maturation-Dependent GABAA Receptor Expression and Pharmacological Activation in iPSC-Derived DRG Neurons
GABAA receptor expression increased during neuronal maturation, with over 90% of DRG neurons exhibiting functional responses from week 2 onward (left), indicating reliable ligand-gated ion channel functionality for screening workflows. Representative current traces (right) demonstrate dose-dependent inward currents evoked by increasing GABA concentrations, confirming receptor responsiveness and assay suitability for pharmacological profiling.
Electrophysiology Excitability and Neuronal Firing
RealDRGx™ neurons exhibited increasing excitability over time, including spontaneous and evoked firing that was sensitive to sodium channel blockade—highlighting their functional maturity and suitability for screening. Key electrophysiological features included:
- Resting membrane potential (RMP): Averaged between −40 to −50 mV
- Spontaneous firing: Frequency increased over the maturation period
- Evoked firing: Reliable responses to current injection from 0 to 200 pA
- TTX sensitivity: Clear inhibition of firing at nanomolar concentrations
- Progressive excitability: Enhanced firing behaviour from week 1 to 6
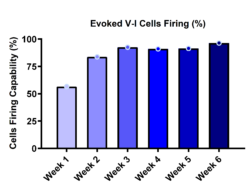
Progressive Increase in Evoked Firing Capacity

TTX-Sensitive Action Potential Firing
Representative voltage traces show evoked action potentials under control conditions and following application of 500 nM TTX. The clear inhibition confirms functional sodium channel activity and the platform’s suitability for screening excitability-modulating compounds.
A Validated, Scalable Platform for Ion Channel-Targeting Pain Drug Discovery
Our iPSC-derived DRG neuron electrophysiology platform offers:
- A scalable, human-relevant alternative to primary DRG models
- Automated, high-throughput compatibility using the SyncroPatch 384i
- Pharmacological validation across key ion channels and receptors
- Functionally mature sensory neurons, ideal for pain-focused drug discovery

